The following symbols may be used on FUJIFILM Sonosite products, packaging, and containers. This list is comprehensive for all of our products. All products do not display all of the following symbols.
| Symbol | Symbol Title | Symbol Description | Standards Reference |
|---|---|---|---|
 | Manufacturer | Indicates the medical device manufacturer. | ISO 15223-1 Medical devices – symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements 5.1.1 |
 | Date of manufacture | To indicate the date on which a product was manufactured. | ISO 7000- Graphical symbols for Use on Equipment 5.1.3 |
| Catalog Number | Indicates the manufacturer’s catalogue number so that the medical device can be identified. | ISO 15223-1 Medical devices – symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements 5.1.6 | |
| Serial Number | Indicates the manufacturer’s serial number so that a specific medical device can be identified. | ISO 15223-1 Medical devices – symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements 5.1.7 | |
| Batch code, date code, or lot code type of control number | Indicates manufacturer's batch code so that the batch or lot can be identified. | ISO 15223-1 Medical devices - Symbols to be used with medical device labels, labeling and information to be supplied - Part 1: General Requirements 5.1.5 | |
 | European community authorized representative | Indicates the authorized representative in the European community. | ISO 15223-1 Medical devices - symbols to be used with medical device labels, labeling and information to be supplied 5.1.2 |
 | Conformité Européene Notified Body Reference No.: 2797 | Indicates European technical conformity and identification of notified body responsible for implementation of the procedures set out in Annexes II, IV, V, and VI. | _ |
 | CE marking | Signifies European technical conformity. | _ |
 | UK Conformity Assessed | Mark that indicates conformity with the applicable requirements for products sold within Great Britain. | The Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019. |
 | UK Conformity Assessed with approved Body Number | Mark, including the approved body number, that indicates conformity with the applicable requirements for products sold within Great Britain. | The Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019. |
 | Medical Device | Indicates the item the label is adhered to is categorized as a medical device per the MDR, Annex 1, 23.2, q. | EU MDR EU MDR Annex I, 23.2 (q) |
 | UDI | Unique device identifier. This symbol is optional and is used when there are multiple data carriers on a label. | ISO 15223-1:2021 5.7.10 |
 | Caution | Indicates that caution is necessary when operating the device or control close to where the symbol is placed. | |
 | Refer to instruction manual/booklet | Follow instructions for use (used in accordance with IEC 60601-1). | IEC 60601-1 Medical electrical equipment Part 1: General requirements for basic safety and essential performance D.2-10 |
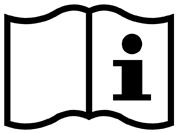 | Consult instructions for use | Indicates that the operating instructions should be considered when operating the device or control close to where the symbol is placed. | ISO 15223-1 Medical devices – symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements 5.4.3 |
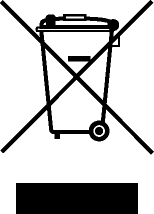 | Recycle: Electronic Equipment | Do not throw in trash. | BS EN 50419 Marking of Electrical and Electronic Equipment in accordance with Directive 2012/19/EU for the Waste of Electrical and Electronic Equipment (WEEE) and Directive 2006/66/EC on Batteries and Accumulators and Waste Batteries and Accumulators Annex IX |
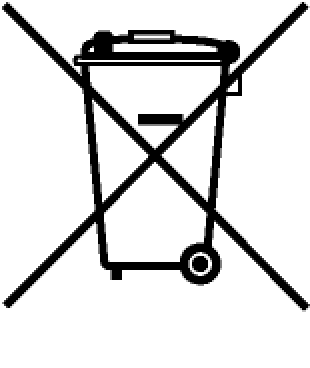 | Historic WEEE | Historic WEEE (trash can) without bar. No bar signifies products made before 2005. | _ |
 | Electrostatic sensitive devices | Indicates packages containing electrostatic sensitive devices, or identifies a device or a connector that has not been tested for immunity to electrostatic discharge. | IEC 60417 Graphical Symbols For Use On Equipment 5134 |
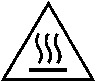 | Caution hot | Indicates that the marked item can be hot and should not be touched without taking care. | ISO 7000 / IEC 60417 Graphical symbols for use on equipment 5041 |
 | ETL (electronic Testing Laboratories) certification mark | Proof product complies to North American safety standards. | _ |
 | UL Classified Canada and US | Certification mark. | |
 | UL recognized component part | UL recognized component mark for Canada and the United States | _ |
 | Canadian Standard Association Certification Mark | CSA certification mark signifying that the product complies with the applicable CSA and ANSI/UL requirements and is authorized for use in Canada and the US. | _ |
 | TUV Rhineland of North America | TUV Rhineland of North America. The “C” and US” indicators signify that the product has been evaluated to the applicable CSA and ANSI/UL standards for use in Canada and the US, respectively. | _ |
 | TUV, SUD, Canada & USA | TUV mark applied to labels where the product has been certified to Technischer Überwachungsverein. "C" demonstrates an electrical product has been successfully tested and certified by a Certification Body accredited by the Standards Council of Canada (SCC). | _ |
 | InMetro (Brazil) TUV | Brazilian equipment and electrical components approval | _ |
 | InMetro (Brazil) NCC | Signifies product has been assessed and certified to ABNT ISO/IEC 17065 and ABNT ISO / IEC 17021-1. | _ |
 | China Compulsory Certificate mark (“CCC Mark”) | A compulsory safety mark for compliance to Chinese national standards for many products sold in the People’s Republic of China. | _ |
 | China Pollution Control (10) | Pollution Control Logo. (Applies to all parts/products listed in the China RoHS disclosure table. May not appear on the exterior of some parts/products because of space limitations.) | ISO 7000: Graphical symbols for use on equipment 1135 |
 | Symbol, China RoHs, color-black | China RoHs "e" indicates that the product contains less than the maximum concentration value of six hazardous substances. | _ |
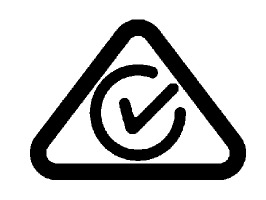 | Regulatory Compliance Mark (RCM) | Indicates C-Tick-Regulatory Compliance Mark for Australia and New Zealand Device complies with relevant Australian and New Zealand regulations for electronic devices. | AS/NZS3820 |
 | Non-ionizing electromagnetic radiation | To indicate generally elevated, potentially hazardous, levels of non-ionizing radiation, or to indicate equipment or systems e.g. in the medical electrical area that include RF transmitters or that intentionally apply RF electromagnetic energy for diagnosis or treatment. | IEC 60417 |
 | Federal Communications Commission (FCC) Declaration of conformity | FCC—Tested to Federal Communications Commission requirements. Device complies with relevant FCC regulations for electronic devices. | Federal Communications Commission (FCC) Declaration of conformity 21 Part 15 |
 | Magnetic resonance environment unsafe | Indicates the system is an item that is known to pose hazards in all MR environments. | ASTM International (American Society for Testing and Materials) ASTM F2503 |
 | Degrees of Protection Provided by Enclosures (IP Code). | Protected against the effects of temporary immersion in water. | IEC 60601-1 Medical electrical equipment Part 1: General requirements for basic safety and essential performance D.3-2 |
 | Degrees of Protection Provided by Enclosures (IP Code). | Protected against solid objects over 12mm and direct sprays of water up to 15 degrees from the vertical. | IEC 60601-1 Medical electrical equipment Part 1: General requirements for basic safety and essential performance D.3-2 |
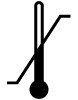 | Temperature limit | Indicates the temperature limits to which the medical device can be safely exposed. | ISO 15223-1 Medical devices – symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements 5.3.7 |
 | Humidity limitation | Indicates the range of humidity to which the medical device can be safely exposed. | ISO 15223-1 Medical devices – symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements 5.3.8 |
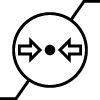 | Atmospheric pressure limitations | Indicates the range of atmospheric pressure to which the medical device can be safely exposed. | ISO 15223-1 Medical devices – symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements 5.3.9 |
| Fragile handle with care | Indicates a medical device that can be broken or damaged if not handled carefully. | ISO 15223-1 Medical devices – symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements 5.3.1 | |
| Keep dry | Indicates a medical device that needs to be protected from moisture. | ISO 15223-1 Medical devices – symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements 5.3.4 | |
 | Corrugated recycles | Shipping box is made of corrugated cardboard and should be recycled accordingly. | _ |
 | RESY – Recycling Symbol | Paper recycle. | _ |
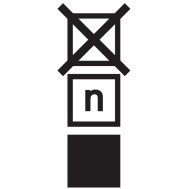 | Stacking limit by number | Do not stack over n high, where n represents the number on the label. | ISO 7000 Graphical symbols for use on equipment |
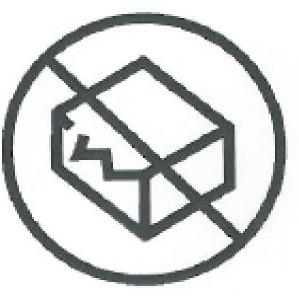 | Do not use if packaging is damaged | Do not use product if the packaging has been damaged. | ISO 7000-2606 ISO 15223-1, Clause 5.2.8 |
 | Maximum weight load | Indicates total weight of the equipment, including the safe working load. | IEC 60601-1 Medical Electrical Equipment Part 1: General requirements for basic safety and essential performance 7.2.21 |
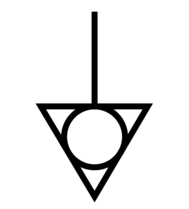 | Potential equalization terminal | Identifies potential equalization terminal. | IEC 60601-1 Medical Electrical Equipment Part 1: General requirements\nfor basic safety and essential performance D.1-8 |
 | Defibrillation-proof type CF applied part | Identifies a defibrillation-proof type CF applied part complying with IEC 60601-1. | IEC 60601-1 Medical electrical equipment Part 1: General requirements for basic safety and essential performance D.1-27 |
 | Type BF applied parts | Identifies type BF applied part complying with IEC 60601-1. | IEC 60601-1 Medical Electrical Equipment Part 1: General requirements for basic safety and essential performance D.1-20 |
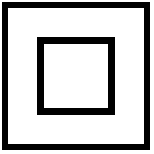 | Class II Equipment | To identify equipment meeting the safety requirements specified for Class II equipment according to IEC 60536. | IEC 60601, table D1-9 |
 | Warning; Biological hazard | To warn of biological hazard. | ISO 7010 - Graphical symbols -- Safety colors\nand safety signs W009 |
 | Warning; Laser beam | To warn of a laser beam. | ISO 7010-W004 |
 | Alternating current | Indicates on the rating plate, that the equipment is suitable for alternating current only, in order to identify appropriate terminals. | IEC 60601-1 Medical electrical equipment |
| Direct current (DC) | To indicate on the rating plate that the equipment is suitable for direct current only; to identify relevant terminals. | IEC 60601-1 Medical electrical equipment | |
| N/A | Indicates follow manufacturer’s instructions for disinfecting time. | _ | |
| N\/A | Indicates disinfect transducer. | _ | |
| N\/A | Indicates handle with care. | _ | |
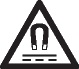 | Caution, static magnetic field hazard | Identifies areas with potentially hazardous static magnetic fields and forces in an installation. | IEC 60417-6204 |
 | Power Button | Power button symbol. | - |
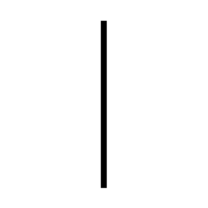 | "ON" (power) | To indicate connection to the mains. | ISO/IEC 13251 |
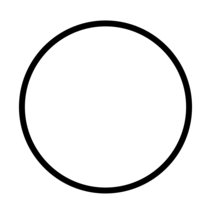 | "OFF" (power) | To indicate disconnection from the mains. | ISO/IEC 13251 |
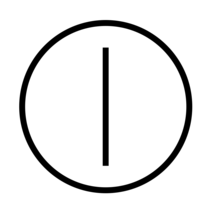 | "ON" / "OFF" | To indicate connection to or disconnection from the mains. | IEC 62288 |
 | General Prohibition Sign | To signify a prohibited action. | ISO 20023 |
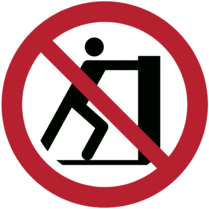 | No pushing | To prohibit pushing against an object. | ISO 24409-2:2014 |
 | Gel | - | - |
 | Sterilized using irradiation | Indicates a medical device that has been sterilized using irradiation. | ISO 15223-1 Medical devices - Symbols to be used with medical device labels, labeling and information to be supplied - Part 1: General Requirements 5.2.4 |
 | Sterilized using ethylene oxide | Indicates a medical device that has been sterilized using ethylene oxide. | ISO 15223-1 Medical devices Symbols to be used with medical device labels, labeling and information to be supplied - Part 1: General Requirements\n5.2.3 |

